FDA approves the new GE’s Signa Premier 3.0T MRI scanner
The GE Healthcare Company is proud to present the new SIGNA Premier 3.0T MRI machine with a wide gantry aperture now available on the US market. SIGNA Premier MRI scanner is the result of the joint four-year cooperation of GE with the United States National Football League (NFL) and research centers. Their consolidated efforts were directed at creating and improving imaging devices which could help scientists detect and potentially diagnose mild Traumatic Brain Injury (mTBI). The new magnetic resonance imaging machine SIGNA Premier ensures high-precision diagnostic results providing excellent technical capabilities which can be focused on medical investigations in neurology and oncology in particular.

Signa Premier 3.0T MRI scanner review
Eric Stahre, President and CEO of Global Molecular Imaging (MI) and Computed Tomography (CT) within GE Healthcare, said, "We are thrilled to bring SIGNA Premier to clinicians. We believe that its advanced applications and breakthrough innovations will deliver research-focused clinical capabilities and wide-bore patient comfort. This new system will help clinicians push the boundaries of what’s possible with MR."
GE Healthcare is really proud of the fact that the new Signa Premier 3.0T has been approved and has received a certificate from the Food and Drug Administration (FDA) of the United States Department of Health and Human Services (HHS).

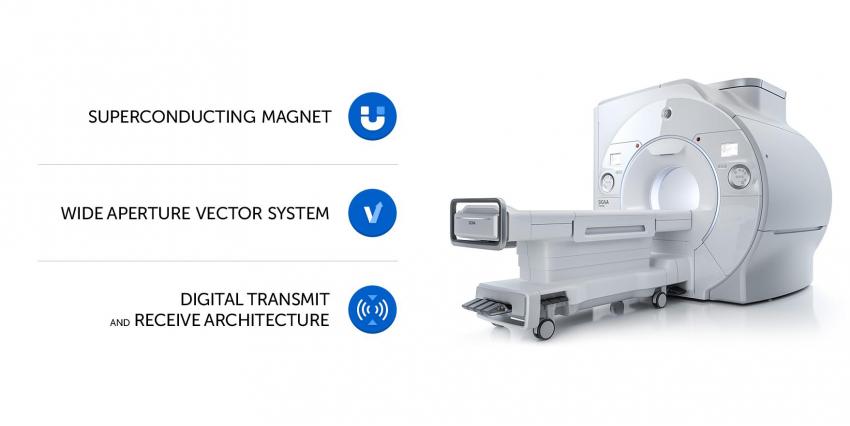
SIGNA Premier 3.0T includes:
- new improved GET superconducting magnet;
- vector system which is the most powerful GE's development among 3.0T systems with a wide aperture;
- modern, powerful digital radio-frequency transmit and receive architecture.
The magnetic resonance imaging scanner of this kind will be very beneficial for clinical and research application as it is the high-performance equipment possessing machine learning software that also includes cloud analytics. SIGNA Premier is equipped with the innovative SuperG vector technology. The SuperG gradient coil serves to deliver ultra-high efficiency performance and is notable for outstanding effectiveness and stability. Signa Premier also is equipped with improved Human Connect protocols (MultiShell DTI and high-quality fMRI), receiving higher resolution images without compromising the patient comfort or the gantry aperture size. In summary, the Signa Premier MRI scanner turned out to be a unique device possessing the 60-centimeter MRI machine functionality but with a 70-centimeter aperture.
The new radio frequency technology allows obtaining patient scan data simultaneously from multiple surface coils using 146 separate sensor channels which, at the same time, provide more intensive scanning, higher image quality, and overall enhanced clinical efficiency.
Comfortable brain diagnostic procedures for all patients
The GE Company has developed a 48-channel head coil which guarantees remarkable performance for practically every patient. An adjustable coil design is suitable for 99.99 percent of the population including those patients who have extremely large heads and short necks. At the same time, high signal-to-noise ratio (SNR) and excellent image quality remain unchanged. In addition, the SIGNA Premier scanner is able to perform a quick brain scan within five minutes, using HyperSense — a new scanning instrument ensuring the eight-time faster examination routine and being part of the HyperWorks application suite.

We can go on discussing all the advantages of this scanner for a long time because the GE Healthcare Company manufactures truly top-class diagnostic equipment. We are sure you will see it quite soon for yourself more than once. Visiting our medical equipment trading platform BiMedis, you will always be able to view numerous equipment options presented by well-known brands such as GE, Toshiba, Siemens, Hitachi, Philips, etc.
09.02.2018